Author

ABSTRACT
Globally saline lakes serve as important habitats for migrating shorebirds and water birds globally most being sites of internationally important wetlands and RAMSAR sites with highly productive ecosystems. This article researches and outlines six environmental pressures faced by saline-alkaline lakes globally; some that are increasing in strength over time. Primary salinization and anthropogenically-induced secondary salinization are one of the most harmful environmental pressures on saline lakes. Furthermore surface freshwater inflow diversions cause floodplain erosion leading to irreparable ecological damage. Recently climate and atmospheric variations have lead to longer periods of dryness causing local biota evolution to withstand extended desiccation periods. This paired with biological interruptions from invasive aquatic species leads to long-term ecological damage and lowered biodiversity. The impacts of mining were found to be detrimental in terms geological disturbance of the surrounding lake perimeter however in some cases, mined lakes have been used as recreational spaces as a mitigation strategy. Finally industrial and agricultural waste dumping and groundwater leachate has gained traction in the last decade predicted to cause elevated levels of damage unless controlled through stricter legislation. This article is only able to provide a snapshot of the current ecological pressures and further long-term research and protection needs to be carried out for this delicate yet important lake type. Mitigation strategies should be improvements in eco-tourism, land use control, stricter laws and drawback of intensive agricultural farming amongst others, which can be implemented with immediate effect.
Keywords: Alkaline-Saline Lakes, Ecological Pressures, Climate Change, Flora-Fauna Diversity, Reduced Biodiversity.
1. INTRODUCTION
Lakes are defined as a large expanse of water enclosed by land, with rivers and streams, being the only intermediaries to the sea (Cambridge, 2017). Kirillin & Shatwell, (2016) define lakes into 2 distinct types; thermally stratified and mixed stratified, respectively. Most lakes (~70%) are situated along the tropical zone (0ºC ± 23.5ºC), with 8 million lakes > 1 ha in size (Lehner & Döll, 2004). Lake surface temperatures (optimal temperature = 20ºC), influenced by solar radiation, alter the key chemical, biological and physical mechanisms of a lake (Sharma et al., 2015) by inducing phytoplankton productivity, being inversely correlated to the lake depth.
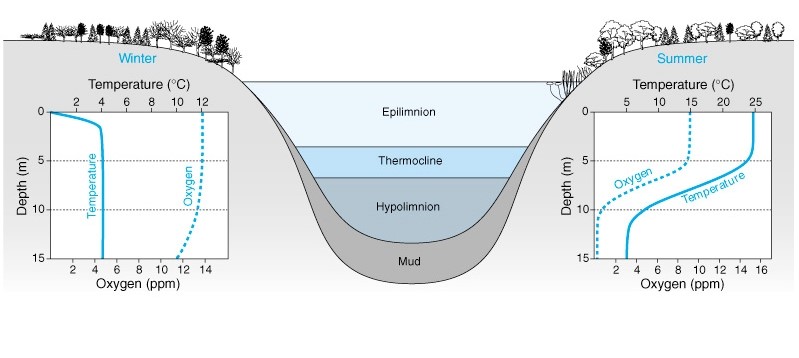
Figure 1: The three-layered structure of a thermally stratified lake, made of the Eplimnion, the Metalimnion and the Hypolimnium. A direct relationship is observed between Oxygen concentrations (ppm) and Depth (m), with a constant thermocline in the winter however an inverse relationship is seen between Oxygen Concentrations (ppm) and Temperature (ºC) in regard to Depth (m) in the summer (Lentic vs. Lotic Systems, n.d.)
Overall more abundant thermally stratified lakes, explained by Shahidi & Imberger (2001) have a primary three-layer structure (Figure 1); the Eplimnion (a warm surface-mixed oxic upper layer), the Metalimnion (thermocline separating the upper and lower layer) and the Hypolimnion (a cold-deep anoxic lower layer), with the stratification being a crucial factor influencing the distribution of total dissolved and suspended particles.
Primarily being closed drainage (endorheic) systems in comparison to terrestrial streams and marine settlements, Gottschalk & Kahlert (2012) identify lakes to be transcendent environmental change sensors due to the “littoral diatoms” present. Diatom assemblages were found to be mainly influenced by the pH gradient, when tested in the Swedish lakes. Being able to alter regional climates caused by the exchange of heat and water with the atmosphere (Krinner, 2003), Tranvik et al., (2009) identify lakes to be important sites for the transport, storage and transformation of ample loads of Dissolved Inorganic Carbon (DIC), Dissolved Organic Carbon (DOC), Particulate Inorganic Carbon (PIC) and Particulate Organic Carbon (POC).
However due to the recent impacts of exceeding anthropogenic use on sensitive lake systems, a new branch of the least-studied lake type has been distinctly recognized for its importance and high endemism; meromictic lakes e.g. soda lakes, saline-alkaline lakes (Boehrer & Schultze, 2008). Thackeray et al., (2006) show these lakes to have developed permanent stratification where vertical stratification is absent throughout the annual cycle due to a stagnant bottom layer, specifically impacting the redistribution of dissolved substances and the lake biocenosis. Their importance as pristine habitats preserving early life fossils and a source for “biomolecules with biotechnological potential” is only just being recognized (Antony et al., 2013).
1.1 Development of Saline-Alkaline Lakes: Key Characteristics
As pointed out by Oduor & Schagerl (2007), saline-alkaline lakes are frequent in dry, arid (25 – 200 mm annual precipitation) and semi-arid (200 – 500 mm annual precipitation) endorheic regions globally (Figure 2), where evaporation > precipitation (Williams, 2002). They are considered as one of the most productive lake systems globally (Ogato et al., 2015). Inland saline lakes have salinity levels of > 3 g L-1, are strongly alkaline (pH > 10) and have a worldwide volume of ~85,000 km3 (Tweed et al., 2011), meromictic in nature. They are particularly perceptive to uncertain variations in weather and climate, despite being typically small-scale (< 50 ha) and relatively shallow (~ 1m), (Kovács et al., 2014) with their physical and chemical features influenced by regional geochemistry, geomorphology and local basin type.
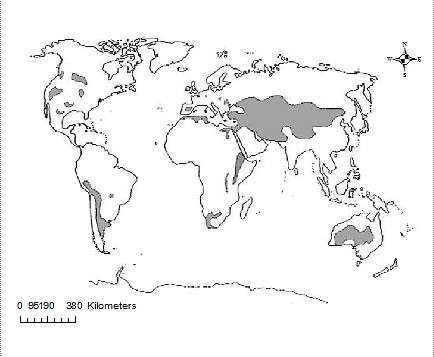
Figure 2: Distribution of salt lakes globally (grey shaded areas), (Williams, 2002]
According to Last (2002), saline lake are classified into systems, in relation to their salinity concentrations, freshwater lakes having the lowest salinity concentrations of < 1 ppm and hypersaline lakes having the highest salinity concentrations of > 50 ppm.
They serve as important habitats for migrating shorebirds and water birds globally (Moore, 2016), most being sites of internationally important wetlands and RAMSAR sites with highly productive ecosystems e.g. Lake Elmenteita, Kenya (Ramsar, 2005). The primary geochemistry of alkaline saline lakes is strongly influenced by alkaline earth metals (Mg2+ and Ca2+) and alkali metals (Na+ and K+), (Witherow & Lyons, 2011), caused by run-off generated from the weathering of local indigenous volcanic geology (Yuretich, 1982). As pointed out by Jellison et al., (2004), saline alkaline lakes are especially abundant in bicarbonate (HCO3-) ions and carbonate (CO32-) ions, often characterized with “low species diversity but high populations of aquatic organisms”.
The basic stratification of saline alkaline lakes is a consequence of “stable gradients in physiochemical properties” (Baatar et al., 2016), and consists of two primary zones; the upper oxygenic zone called the Mixolimnion (0 – 8 m), (Aquatic Lakes, n.d.), further branched in to three sub-zones; Epilimnion, Metalimnion and Hypolimnion and the lower anoxic zone called the Monimolimnion (10 – 20 m), which are divided by a transition zone called the Chemocline (8 – 10 m), (Telemante et al., 2011).
2. DISCUSSION
2.1 Environmental Pressures on Saline-Alkaline Lakes
Despite limited definitive research on environmental pressures affecting salt lakes globally, the section below highlights 6 key anthropogenic-induced pressures and hence subsequent impacts on the lake type and its surrounding habitat.
2.1.1 Salinization
Verschuren et al., (2000) recognizes salinization as one of the main environmental factors that “regulate aquatic community structures” in saline alkaline lakes. There are two main pathways for salinization; primary and secondary, respectively. Williams (2002), defines primary salinization, restricted mostly to endorheic lakes in the arid and semi-arid environments, to be the advancement of the salt particles towards the lake surface lead by capillary action following an increase in the water table, with the prevailing high rates of evaporation leading to salt deposition. On the other hand, secondary salinization is caused by anthropogenic-induced activities such as clearing vegetation on the lake edge and changing land use (Williams, 1999) and is identified as a key impact especially on temporary salt lakes.
Salama et al., (1999) identify groundwater as the “main geological agent for transmitting, accumulating and discharging salt”. Research conducted by Nielsen et al., (2003) on salinization effects on Australian salt lakes clearly indicated severe impacts for local biota following a 1000 mg L-1 increase in salinity (Figure 3), with a 95% probability of killing the biota.
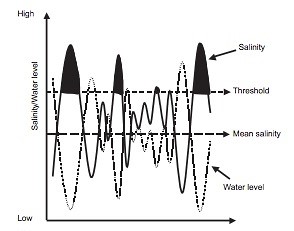
Figure 3: Predicted salinity level fluctuations in relation to water level fluctuations and consequent impacts on local biota in Australian salt lakes. Crossing the threshold of 1000 mg L-1 (black coloured curve area) severely reduced the populations of sensitive non-tolerant biota in the immediate area (Nielsen et al., 2003)
2.1.2 SURFACE FRESHWATER INFLOW DIVERSIONS.
Termed as the most damaging activity pressuring salt lakes (Williams, 2002; Shadkam et al., 2016), surface freshwater inflow diversion has lasting negative impacts. This is the diversion of natural incoming freshwater e.g. rivers, streams for anthropogenic use, causing lake size alterations (Jellison et al., 2004).
A study by Kingsford (2000) on the effect of human-induced surface inflow diversions on Australian floodplains caused by damming river courses, highlights a 40% loss of floodplain area, causing irreparable biodiversity damage. The infamous case of the Aral Sea Crisis (Whish-Wilson, 2002), that forfeited its lake volume by > 80% over 43 years (1960-2003) due to diversion of the inflowing Amu Dar ́ya and the Syr Dar ́ya rivers (UNEP, 2008), respectively, devastated the local ecology, leaving ~3.6 million hectares of seabed exposed (Small et al., 2001). Another recent study by Moore (2016) reported desiccation of Lake Abet in Oregon, USA for agricultural purposes, decreased its volume to a third (236 ha) of the original size and “destroyed the brine shrimp and brine fly populations”, none surviving above a 20% rise in salinity levels, and reduced shorebird numbers by half.
A high impact study on Lake Mono, a saline alkaline lake in California, U.S.A., also demonstrated lowering of the lake level (Weins et al., 1993) by ~ 100m, exposing 12,700 acres of seabed and resulted in a 39m reduction in elevation (“About Lake Mono”, 2017).
2.1.3 Climate and Atmospheric Variations
According to Kovacs et al., (2014), saline alkaline lakes are one of the most sensitive lake types to get affected by climatic and atmospheric variations (changes to hydrological budgets; especially in precipitation levels) due to the absence of periodic flushing activities, which neutralize effects of evapo-concentration (Evans & Prepas, 1996). As concurrent to IPCC Climate Change modeling data on arid and semi-arid areas (IPCC, 2001), Williams (2002) points out unsteady evaporation and precipitation rates to have the most compelling effects on this lake type, with fluctuations in aridity to cause shifts in lake type. He also suggests that rising aridity will cause temporary salt lakes to stay drier for a lengthened period of time, causing local biota evolution to withstand extended desiccation periods and permanent salt lakes will decrease in size but conversely increase in salinity concentrations.
A study by Sui et al., (2016) on the saline alkaline lakes of Songnen Plain, China indicated varying climatic conditions e.g. dry springs and long winters, caused the lakes to shift to a temperate lake type leading to decreases of ~35% in phytoplankton biomass, ~10% in cell diameter and ~5% diatom density at low salinity concentrations (< 3×103 mg/L), respectively.
Research using a developed catchment-scale hydrological model on Lake Abiyata, Ethiopia showed that a 4% decline in precipitation levels on the catchment area lead to a 14% decrease in river runoff and vice versa. Land use alterations caused an approximate 50% decrease in the catchment area and deforestation, with a drop of 8% in the total river discharge (Legesse et al., 2004). This indicates devastating changes for a very sensitive environment.
2.1.4 Biological Imterruptions
Aquatic invasive species in saline alkaline lakes often alter the core ecology of the lake, by placing direct pressure on limited food resources (Jackson & Mandrak, 2002), and eventually driving out the native species. Rahel (2007) suggests anthropogenic activity e.g. dam and canal constructions, global shipping routes and accidental introduction to be major sources of alien species into these lakes.
Research by Williams (2002) stated certain Canadian and Bolivian lakes, who encouraged “continual restocking” of certain invasive fish species, for socio-economic purposes e.g. fisheries; as most would be killed off due to non-tolerance to the harsh environmental conditions, to severely impact the native fish populations through over-competition for resources. Again, the Aral Sea was highlighted, with claims of at least 21 fish species being introduced in 1927 for socio-economic purposes, now non-existent in the lake.
However a study by Welicky et al., (2017) on the effect of drought on Anchor worm (Lernaea cyprinacea) abundance in Mozambique tilapia in hypersaline lakes showed a positive effect, with the drought completely eradicating this freshwater invasive species due to its inability to tolerate such increased salinity levels. Another similar case study of the Lake Bullen Merri and several hypersaline lakes in Victoria, Western Australia by Timms (2005), stated the growth of the invasive eels in these waters without any distinct negative effect, which are now accounted for as native species.
2.1.5 Mining
With most saline alkaline lakes representative of storing economically valuable minerals e.g. gold and nickel near many saline playas in Western Australia, creation of access roads on lake beds lead to the geological disturbance of the surrounding lake perimeter (Timms, 2005). A study of the effects of gold mining at Lake Carey by Datson, (2009) after a 10-year period, revealed the discharge of “hypersaline groundwater from mine dewatering-extra salt load” to extend its hydroperiod, a possible drawdown effect on the destabilization of the lake sediments due to heavy metal leaching, leading to an almost 90% reduction in vegetation showing no inclination of further increasing and a substantial decrease in species biodiversity.
As pointed by Hancock et al., (2004), open cast mining often leads to the formation of a void remaining at the end of the mine’s operational stage which, in most cases, does not get filled. The study, focusing on the changes in salinity levels of a “post-mining landscape final void” (Figure 4) in Hunter Valley, Australia found an approximate four-fold increase in salinity levels, from 2,453 mg l-1 to 8,909 mg l-1, over five decades, caused by the soil seepage and runoff as well as the groundwater intrusion.
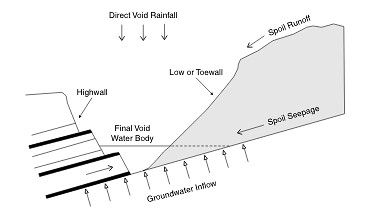
Figure 4: A schematic diagram indicating all the water and salt input and outputs in a “post-mining landscape final void” (Hancock et al., 2004)
On the other hand, 8 similarly mined lakes in the Collie Basin region in Western Australia have been converted and utilized as recreational places e.g. wildlife conservation and aquaculture among others, after appropriate remediation procedures (Doupé & Lymbery, 2005), however unable to attain the original biodiversity.
2.1.6 Pollution
The dominant source of saline lake water pollution is often understood to be industrial and agricultural waste dumping and groundwater leachate, especially in heavily farmed areas around the lake (Williams, 2002). The concept of dumping wastes in these lakes is often found to be acceptable due to their low economic viability, comparative to other lake types e.g. freshwater lakes. Jackson & Kulecho, (2008), study a classic example of Lake Nakuru, an endorheic hypereutrophic saline alkaline lake in Kenya, East Africa, into which an estimated 178 small-to-medium scale industries have been known to dump a variety of products e.g. batteries, paints, detergents and plastics among others. Results indicated a three-fold increase in toxic trace metals and heavy metals (Table 1) when compared to levels from previous studies. Direct impacts were: some lake areas having salinity levels of < 10 mS, killing off the crucial cyanobacteria Spirulina platensis hence a ~30% Lesser Flamingos (Phoeniconaias minor) mortality rate.
Uncontrolled dumping of sewage and industrial effluents has a very debilitating ecological effect on saline-alkaline lakes. Research by Pérez-Fuentetaja et al. (2010) on the yet-occurring effects of polychlorinated biphenyls (PCB) and polybrominated diphenyl ethers (PBDEs) in wild common carp in Lake Erie, USA indicated harmful interruptions to the endocrine systems in the carp, with ‘neurobehavioral deficits’ and carcinogenic capabilities. Sources for the high PCB and PBDE levels in carp were traced back to solid waste disposal (particularly electronic wastes) from surrounding urban settlements. Despite PCB and PBDE being banned from the 1970’s in USA, its impacts continue to wreck havoc on delicate organisms in fragile saline-alkaline lake ecosystems.
Furthermore excess chemical leakage into soda lakes due to agricultural run off is another significant contributor to ecosystem disruption. Despite the remote geographical location of Lake Bogoria, Kenya; Bettinetti et al., (2011) determined moderate contamination of a harmful pesticide, DDT, across the lake, however individually discovering a high concentration ratio of 1:1 pp’DDE to pp’DDT contamination in the Acacia leaves (Acacia tortillis), collected from the lake edge perimeter. Despite being banned globally, DDT is still used in Africa and this region for the fight against malaria. The study suggested “aerial transport or dust” originating from on-site pesticide storage facilities to be the source. The fluctuations in lake water chemistry due to harmful pollutant addition has led to increased cyanobacterial toxicity. This in turn incited high fatality rates in Lesser Flamingo (Phoeniconaias minor) populations in the last decade, up to 700 birds day-1 (Harper et al., 2003), due to ingestion of phytoplankton containing cyanobacterial toxins (0.196 μg g-1 per fecal pellet).
However, a study by Kanekar et al., (1998) successfully used bioremediation techniques involving some alkaliphilic bacteria species, “Arthrobacter, Bacillus cereus, Citrobacter freundii, Micrococcus agilis and Pseudomonas putida biovar B” to remove phenol, a toxic industrial pollutant, by phenol enrichment of lake sediment samples under laboratory conditions (pH 10, phenol concentrations = 500 mg/l, temperature = 28 ± 2 °C). Results showed a 100% phenol removal with alkaliphilic bacteria having a pH range of ~9.95 – 10.1 and 368 – 600 mg/l phenol content range.
CONCLUSION
The new branch of the least-studied lake type has been distinctly recognized for its importance, chemically harsh lake waters and high endemism; meromictic lakes e.g. soda lakes, saline-alkaline lakes. Despite being recognized as one of the most productive lake systems globally, they continue to face many ecological impacts that continue to increase in intensity.
As repeatedly emphasized (Williams, 1999; Williams, 2002; Antony et al., 2013; Rose 2017), the true ecological importance regarding the functionality of saline alkaline lakes’ physico-chemical characteristics that directly and indirectly influence their overall productivity, is yet to be concisely and extensively researched and determined. Lack thereof has caused uncontrolled human-induced exploitation of these sensitive endemic lake types, some lakes being irreversibly damaged, with socio-economic benefits of lake use preferred over its ecological importance. Despite the damage, governments and lawmakers still have power and time to form appropriate laws, with the need to be more proactive for any significant protection and conservation projects to be carried out (Williams, 2002). Hence the focus of mitigation strategies should be improvements in eco-tourism, land use control, stricter laws and drawback of intensive agricultural farming amongst others, which can be implemented with immediate effect.
REFERENCES
About Lake Mono. (2017). Available at: http://www.monolake.org/about/stats (Accessed: 11th November 2020).
Aquatic Habitats. (n.d.) Available at: http://w3.marietta.edu/~biol/biomes/lakes.htm (Accessed: 11th November 2020).
Antony, C. P., Kumaresan, D., Hunger, S., Drake, H. L., Murell, C. J. & Shouche, Y. S. (2013). ‘Microbiology of Lonar Lake and other soda lakes’. The International Society for Microbial Ecology (ISME) Journal, 7, pp. 468-476. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578565/pdf/ismej2012137a.pdf (Accessed: 11th November 2020).
Baatar, B., Chiang, P. W., Rogozin, D. Y., Wu, Y. T., Tseng, C. H., Yang, C. Y., Chui, H. H., Oyuntsetseg, B., Degermendzhy, A. G. & Tang, S. T. (2016). ‘Bacterial Communities of Three Saline Meromictic Lakes in Central Asia’. PLoS ONE, 11(3), pp. 1-22. doi: 10.1371/journal.pone.0150847
Bettinetti, R., Quadroni, S., Crosa, G., Harper, D., Dickie, J., Kyalo, M., Mavuti, K & Galassi, S. (2011). ‘A Preliminary Evaluation of the DDT Contamination of Sediments in Lakes Natron and Bogoria (Eastern Rift Valley, Africa)’, Ambio, 40(4), pp. 341-350. doi: 10.1007/s13280- 011-0142-8
Boehrer, B. & Schultze, M. (2008). ‘Stratification of lakes’. Review of Geophysics, 46, pp. 1- 27. doi: 10.1029/2006RG000210
Cambridge. (2017). Lake. Available at: http://dictionary.cambridge.org/dictionary/english/lake (Accessed: 11th November 2020).
Datson, B. M. (2009). ‘Effects of mining on a salt lake in the Western Australia goldfields’. Saline lakes around the world: Unique systems with unique values, 15, pp. 1-2. Available at: http://digitalcommons.usu.edu/nrei/vol15/iss1/45/ (Accessed: 11th November 2020)
Doupé, R. G. & Lymbery, A. J. (2005). ‘Environmental Risks associated with beneficial end uses of mine lakes in Southwestern Australia’. Mine water and the Environment, 24(3), pp. 134- 138. doi: 10.1007/s10230-005-0084-0
Evans, J. C. & Prepas, E. E. (1996). ‘Potential effects of climate change on ion chemistry and phytoplankton communities in prairie saline lakes’. Limnology and Oceanography, 41(5), pp. 1063-1076. doi: 10.4319/lo.1996.41.5.1063
Gottschalk, S. & Kahlert, M. (2012). ‘Shifts in taxonomical and guild composition of littoral diatom assemblages along environmental gradients’. Hydrobiologia, 694, pp. 41-56. doi: 10.1007/s10750-012-1128-7
Hancock, G. R., Wright, A. & Silva, H. D. (2005). ‘Long-term final void salinity prediction for a post-mining landscape in the Hunter Valley, New South Wales, Australia’. Hydrological Processes, 19, pp. 387-401. doi: 10.1002/hyp.5538
Harper, D. M., Childress, R. B. Harper, M. M., Boar, R. R., Hickley, P., Mills, S. C., Otieno, N., Drane, T., Vareschi, E., Nasirwa, O., Mwatha, W. E., Darlington, J. P. E. C. & Escuté-Gastulla, X. (2003). ‘Aquatic biodiversity and saline lakes: Lake Bogoria National Reserve, Kenya’ Hydrobiologia, 500, pp. 259-276. Available at: https://link.springer.com/article/10.1023/A:1024722821407 (Accessed: 16th November 2020).
IPCC. (2001). Climate Change 2001: Impacts, Adaptation and Vulnerability. Available at: http://www.ipcc.ch/ipccreports/tar/wg2/index.php?idp=170 (Accessed: 11th November 2020).
Jackson, R. A. & Kulecho, A. (2008). ‘Lake Nakuru-Kenya: A review of environmental impacts of land use changes’. Proceedings of the TAAL 2007: The 12th World Lake Conference, 1, pp. 2241-2245. Available at: http://www.moef.nic.in/sites/default/files/nlcp/Overseas%20Case%20Studies/Q-76.pdf (Accessed: 11th November 2020).
Jellison, R., Zadereev, Y. S., DasSarma, P. A., Melack, J. A., Rosen, M. R., Degermendzhy, A. G., DasSarma, S. & Zambrana, G. (2004). ‘Conservation and Management challenges of Saline Lakes: A Review of five experienced briefs’. Lake Basin Management Initiative, 1, pp. 1- 28. Available at: http://www.academia.edu/12476046/Conservation_and_management_challenges_of_Saline_l akes_A_review_of_five_experience_briefs (Accessed: 11th November 2020).
Kanekar, P. P., Sarnail, S. S. & Kelkar, A. S. (1998). ‘Bioremediation of phenol by alkaliphilic bacteria isolated from alkaline lake of Lonar, India’. Journal of Applied Mibrobiology, 85(1), pp. 1285-1335. doi: 10.1111/j.1365-2672.1998.tb05291.x
Kingsford, R. T. (2000). ‘Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia’. Austral Ecology, 25, pp. 109-127. doi: 10.1046/j.1442-9993.2000.01036.x
Kirillin, G. & Shatwell, T. (2016). ‘Generalized scaling of thermal stratification in lakes’. Earth-Science Reviews, 161, pp. 179-190. doi: 10.1016/j.earscirev.2016.08.008
Krinner, G. (2003). ‘Impact of lakes and wetlands on boreal climate’. Journal of Geophysical Research, 108(16), pp. 1-18. doi: 10.1029/2002JD002597
Kovács, C. S., Lengyel, E., Buczkó, K, Tóth, F. M., Crossetti, L. O., Pellinger, A., Doma, Z. Z & Padisák, J. (2014). ‘Vanishing worlds: alkaline, saline lakes in Central Europe and their diatom assemblages’. Inland Waters, 4, pp. 383 – 396. doi: 10.5268/IW-4.4.722
Last, W. M. (2002). ‘Geolimnology of salt lakes’. Geosciences Journal, 6(4), pp. 347 – 369. doi: 10.1007/BF03020619
Legesse, D., Coulomb, C. V. & Gasse, F. (2004). ‘Analysis of the hydrological response of a tropical terminal lake, Lake Abiyata (Main Ethiopian Rift Valley) to changes in climate and human activities’. Hydrological Processes, 18, pp. 487-504. doi: 10.1002/hyp.1334
Lehner, B. & Döll, P. (2004). ‘Development and validation of a global database of lakes, reservoirs and wetlands’. Journal of Hydrology, 296, pp. 1-22. doi: 10.1016/j.jhydrol.2004.03.028
Moore, J. N. (2016). ‘Recent desiccation of Western Great Basin Saline Lakes: Lessons from Lake Abert, Oregon, U.S.A’. Science of the Total Environment, 554, pp. 142-154. doi: 10.1016/j.scitotenv.2016.02.161
Nielsen, D. L., Brock, M. A., Rees, G. N. & Baldwin, D. S. (2003). ‘Effects of increasing salinity on freshwater ecosystems in Australia’. Australian Journal of Botany, 51(6), pp. 655-665. doi: 10.1071/BT02115
Odour, S. O. & Schagerl, M. (2007). ‘Phytoplankton primary productivity characteristics in response to photosynthetically active radiation in three Kenyan Rift Valley saline – alkaline lakes’. Journal of Plankton Research, 29(12), pp. 1041 – 1050. doi: 10.1093/plankt/fbm078
Ogato, T., Kifle, D. & Lemma, B. (2015). ‘Underwater light climate, thermal and chemical characteristics of the tropical soda lake Chitu, Ethiopia: Spatial-temporal variations’. Limnologica, 52, pp. 1-10. doi: 10.1016/j.limno.2015.02.003
Pérez-Fuentetaja, A., Lupton, S., Clapsadi, M., Samara, F., Gatto, L., Biniakewitz, R. and Aga, D. S. (2011). ‘PCB and PBDE levels in wild common carp (Cyprinus carpio) from eastern Lake Erie’, Chemosphere, 81(4), pp. 541 – 547. doi: 10.1016/j.chemosphere.2010.06.033
Rahel, F. J. & Olden, J. D. (2008). ‘Assessing the effects of Climate Change on Aquatic Invasive Species’. Conservation Biology, 22(3), pp. 521-533. doi: 10.1111/j.1523- 1739.2008.00950.x
Ramsar. (2005). Lake Elmenteita added to the Ramsar List. Available at: http://www.ramsar.org/news/lake-elmenteita-added-to-the-ramsar-list (Accessed: 11th November 2020).
Rodgers, S. (n. d.). Lentic vs. Lotic Systems. Available at: https://spencerrodgers.weebly.com/lotic-systems-vs-lentic-systems.html# (Accessed: 11th November 2020)
Rose, P. (2017). Africa’s most toxic lakes are a paradise for fearless flamingos – Cosmos. Available at: https://cosmosmagazine.com/biology/africa-s-most-toxic-lakes-are-a-paradise-for-fearless-flamingos (Accessed: 16th November 2020).
Salama, R. B., Otto, C. J. & Fitzpatrick, R. W. (1999). ‘Contributions of groundwater conditions to soil and water salinization’. Hydrogeology Journal, 7, pp. 46-64. doi: 10.1007/s100400050179
Shadkam, S., Ludwig, F., Vliet, M. T. V. V., Pastor, A. & Kabat, P. (2016). ‘Preserving the world second largest hypersaline lake under future irrigation and climate change’. Science of the Total Environment, 559, pp. 317-325. doi: 10.1016/j.scitotenv.2016.03.190.
Shahidi, A. E. & Imberger, J. (2001). ‘Anatomy of turbulence in thermally stratifies lakes’. Limnology and Oceanography, 46(5), pp. 158-1170. Available at:: http://onlinelibrary.wiley.com/doi/10.4319/lo.2001.46.5.1158/full (Accessed: 11th November 2020)
Sharma, S., Gray, D. K., Read, J. S., O’Reilly, C. M., Schneider, P., Qudrat, A., Gries, C., Stefanoff, S., Hampton, S. E., Hook, S., Lenters, J. D., Livingstone, D. M., McIntyre, P. B., Adrian, R., Allan, M. G., Anneville, O., Arvola, L., Austin, J., Bailey, J., Baron, J. S., Brookes, J., Chen, Y., Daly, R., Dokulil, M., Dong, B., Ewing, K., Eyto., E. D., Hamilton, D., Havens, K., Haydon, S., Hetzenauer, H., Heneberry, J., Hetherington, A. L., Higgins, S. N., Hixson, E., Izmest ́eva, L. R., Jones, B. M., Kangur, K., Kasprzak., P., Köster, O., Kraemer, B. M., Kumagai, M., Kuusisto, E., Leshkevich, G., May, L., MacIntyre, S., Müller-Navarra, D., Naumenko, M., Noges, P., Noges, T., Neiderhauser, P., North, R. P., Paterson, A. M., Plisnier, P. D., Rigosi, A., Rimmer, A., Rogora, M., Rudstam, L., Rusak, J. A., Salmaso, N., Smal, N. R., Schindler, D. E., Schladow, G., Schmidt, S. R., Schultz, T., Silow, E .A., Straile, D., Teubner, K., Verburg, P., Voutilainen, A., Watkinson, A., Weyhenmeyer, G. A., Williamson, C.E. & Woo., K. H. (2015). ‘A global database of lake surface temperatures collected by in situ and satellite methods from 1985-2009’. Scientific Data, 2, pp. 1-19. doi: 10.1038/sdata.2015.8
Small, I., Van der Meer, J. and Upshur, R.E.G. (2001). ‘Acting on an Environmental Health Disaster: The Case of the Aral Sea’, Environmental Health Perspectives, 109 (6), pp. 547-549. Available at: https://ehp.niehs.nih.gov/doi/pdf/10.1289/ehp.01109547 (Accessed: 11th November 2020).
Sui, F., Zang, S., Fan, Y. & Ye, H. (2016). ‘Effects of different saline-alkaline conditions on the characteristics of phytoplankton communities in the lakes of Songnen Plain, China’. PLoS ONE, 11(10), pp. 1-18. doi: 10.1371/journal.pone.0164734
Thackeray, S. J, George, D. G., Jones R. I. & Winfield, I. J. (2006). ‘Statistical quantification of the effect of thermal stratification on patterns of dispersion in a freshwater zooplankton community’. Aquatic Ecology, 40(1), pp. 23-32. doi: 10.1007/s10452-005-9121-3
Timms, B. V. (2005). ‘Salt lakes in Australia: present problems and prognosis for the future’. Hydrobiologia, 552, pp. 1-15. doi: 10.1007/s10750-005-1501-x
Tranvik, L.R., Downing, J. A., Cotner, J. B., Loiselle, S. A., Striegl, R. G., Ballatore, T. J., Dillon, P., Finlay, K., Fortino, K., Knoll, L. B., Kortelainen, P. L., Kutser, T., Larsen, S., Laurion, I., Leech, D. M., McCallister, S. L., McKnight, D. M., Melack, J. M., Overholt, E., Porter, J. A., Prairie, Y., Renwick., W. H., Roland, F., Sherman, B. S., Schindler, D.W., Sobek, S., Tremblay, A., Vanni., M. J., Verschoor, A. M., Wachenfedlt, E. V. & Weyhenmeyer, G. A. (2009). ‘Lakes and reservoirs as regulators of carbon cycling and climate’. Limnology and Oceanography, 54(6), pp. 2298-2314. doi: 10.4319/lo.2009.54.6_part_2.2298
Telemante, O. T., Alcocer, J., Beddows, P. A., Briones, E. G. G. & Lugo, A. (2011). ‘The key role of the chemolimnium in meromictic cenotes of the Yucatan Peninsula, Mexico’. Hydrobiologia, 677(1), pp. 107-127. doi: 10.1007/s10750-011-0746-9
Tweed, S., Grace, M., Leblanc, M., Cartwright, I. & Smithyman, D. (2011). ‘The individual response of saline lakes to a severe drought’. Science of the Total Environment, 409, pp. 3919- 3933. doi: 10.1016/j.scitotenv.2011.06.023
UNEP. (2008). Vital Water Graphics – An Overview of the State of the World’s Fresh and Marine Waters (2nd Edition). Available at: http://www.unep.org/dewa/vitalwater/article3.html (Accessed: 11th November 2020).
Verschuren, D., Tibby, J., Sabbe, K & Roberts, N. (2000). ‘Effects of depth, salinity and substrate on the invertebrate community of a fluctuating tropical lake’. Ecology, 81(1), pp. 164- 182. doi: 10.1890/0012-9658(2000)081(0164:EODSAS)2.0.CO;2
Weins, J. A., Patten, D. T. & Botkin, D. B. (1993). ‘Assessing Ecological Impact Assessment: Lessons from Mono Lake, California’. Ecological Applications, 3(4), pp. 595-609. doi: 10.2307/1942093
Welicky, R. L., Swardt, J. D., Gerber, R., Netherlands, E. C. & Smit, N. J. (2017). ‘Drought- associated absence of alien invasive anchorworm, Lernaea cyprinacea (Copepoda: Lernaeidae), is related to changes in fish health’. International Journal of Parasitology: Parasites and Wildlife, 23, pp. 1-9. doi: 10.1016/j.ijppaw.2017.01.004
Whish-Wilson, P. (2002). ‘The Aral Sea environmental health crisis’. Journal of Rural and Remote Environmental Health, 1(2), pp. 29-34. Available at: jrtph.jcu.edu.au/vol/v01whish.pdf (Accessed: 11th November 2020).
Williams, W. D. (1999). ‘Salinization: A major threat to water resources in the arid and semi- arid regions of the world’. Lakes and Reservoirs: Research and Management, 4, pp. 85-91. doi: 10.1046/j.1440-1770.1999.00089.
Williams, W. D. (2002). ‘Environmental threats to salt lakes and the likely status of inland saline ecosystems in 2025’. Environmental Conservation, 29(2), pp. 154-167. doi: 10.1017/S0376892902000103
Witherow, R. A. & Lyons, W. B. (2011). ‘The fate of minor alkali elements in the chemical evolution of salt lakes’. Saline Systems, 7(2), pp. 1-17. doi: 10.1186/1746-1448-7-2
Yuretich, R. F. (1982). ‘Possible Influences upon Lake Development in the East African Rift Valleys’. The Journal of Geology, 90(3), pp. 329-337. doi: 10.1086/628684